What you need to know about COVID-19 testing in the Philippines
By Hannah Mallorca and May Dedicatoria, The Philippine STAR
THE Food and Drug Administration (FDA) has approved 21 Real Time – Polymerase Chain Reaction (RT-PCR) testing kits and nine Rapid Antibody-based kits as of April 3.
FDA director general Rolando Enrique D. Domingo, however, advised against the improper use of rapid test kits. “The rapid test kits will yield a faster result compared to PCR-based kits, but it is important that a trained health professional will evaluate and interpret the results. We have to be very cautious in using these rapid test kits because they measure antibodies and not the viral load itself. The body takes time to develop antibodies and this might give a negative result for patients who have been infected but have not yet developed antibodies. A positive result due to cross reaction with other bacteria or viruses is also possible, which is why a confirmatory PCR based test is still required,” he said.
To guide the public, here are the differences between the RT-PCR and Rapid Antibody-based test kits, according to the Department of Health (DoH).
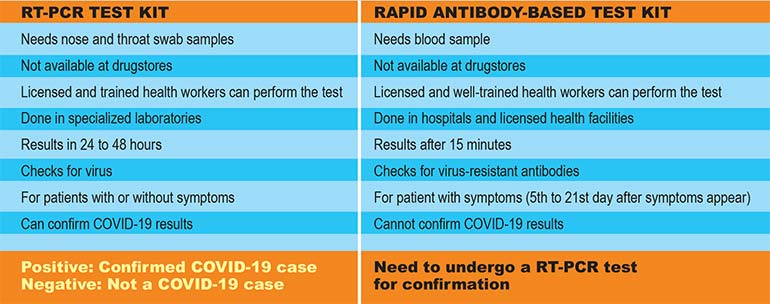
While all Level 2 and 3 hospitals can collect specimen samples, only nine accredited hospitals can conduct RT-PCR tests at present.
According to President Rodrigo R. Duterte’s first Report to the Joint Congressional Oversight Committee regarding the Bayanihan to Heal as One Act, the Department of Science and Technology (DoST) has allotted P53.2 million for the GenAmplifyTM Coronavirus Disease 2019 (COVID-19) rRT-PCR Detection Kit Developed by the University of the Philippines – National Institutes of Health and Manila HealthTek, Inc.
Its successful field validation ran until April 1 while field implementation for 26,000 tests is taking place until April 25 at the Philippine General Hospital, Makati Medical Center, The Medical City, Vicente Sotto Memorial Medical Center, Southern Philippines Medical Center and Baguio General Hospital.
The DoH is also “exploring the possibility of linking lower-level laboratories to those with virus inactivation capacity.”
“Virus inactivation can be done in the more sophisticated laboratories and samples can then be processed inlower-level laboratories. This will achieve the twin goals of increasing testing output per day and ensuring the safety of our health workers,” said Health Secretary Francisco Duque III.
Recently, National Action Plan on COVID-19 chief Carlito Galvez, Jr. said they see massive testing for Patients under Investigation (PUIs) and Patients under Monitoring (PUMs) to start on April 14.
“We are also determined to fast-track the accreditation of substantial laboratories so we can start the massive testing,” he said.
For more #COVID19WATCH contents, visit www.bworldonline.com/covid19watch.


